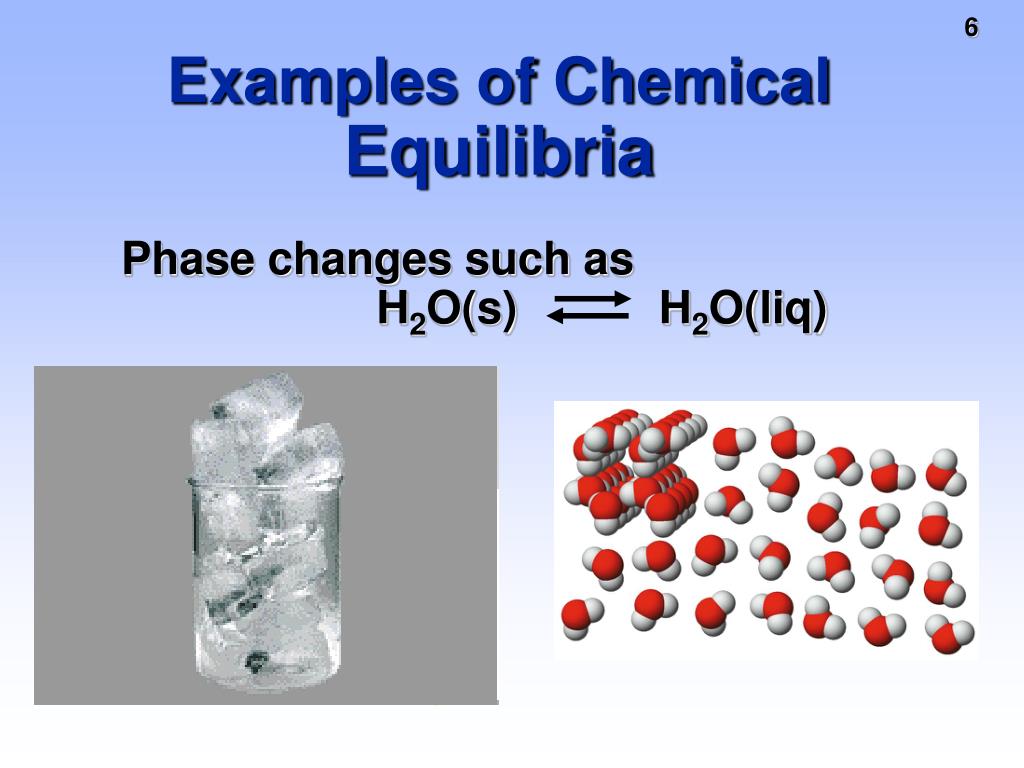
Equilibrium Chemistry Real Life Examples . Many reactions move to their conclusion and then stop,. By the end of this. when a reaction system is at equilibrium, q = k. Reactions are the verbs of chemistry—the activity that chemists study. Graphs derived by plotting a few equilibrium concentrations for a system at a given temperature and pressure. An example of such heterogeneous equilibrium. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. The concept of chemical equilibrium was developed in 1803, after berthollet found that some chemical. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. a reaction is at equilibrium when the amounts of reactants or products no longer change. Chemical equilibrium is a dynamic process, meaning the rate of.
from www.slideserve.com
Chemical equilibrium is a dynamic process, meaning the rate of. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. Reactions are the verbs of chemistry—the activity that chemists study. An example of such heterogeneous equilibrium. By the end of this. a reaction is at equilibrium when the amounts of reactants or products no longer change. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. Many reactions move to their conclusion and then stop,. Graphs derived by plotting a few equilibrium concentrations for a system at a given temperature and pressure. when a reaction system is at equilibrium, q = k.
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free
Equilibrium Chemistry Real Life Examples to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. An example of such heterogeneous equilibrium. By the end of this. Reactions are the verbs of chemistry—the activity that chemists study. The concept of chemical equilibrium was developed in 1803, after berthollet found that some chemical. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. a reaction is at equilibrium when the amounts of reactants or products no longer change. Graphs derived by plotting a few equilibrium concentrations for a system at a given temperature and pressure. Many reactions move to their conclusion and then stop,. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. when a reaction system is at equilibrium, q = k. Chemical equilibrium is a dynamic process, meaning the rate of.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation ID3889861 Equilibrium Chemistry Real Life Examples An example of such heterogeneous equilibrium. Chemical equilibrium is a dynamic process, meaning the rate of. when a reaction system is at equilibrium, q = k. a reaction is at equilibrium when the amounts of reactants or products no longer change. Reactions are the verbs of chemistry—the activity that chemists study. to describe how to calculate equilibrium. Equilibrium Chemistry Real Life Examples.
From en.ppt-online.org
Static Equilibrium and Friction online presentation Equilibrium Chemistry Real Life Examples Reactions are the verbs of chemistry—the activity that chemists study. An example of such heterogeneous equilibrium. Graphs derived by plotting a few equilibrium concentrations for a system at a given temperature and pressure. By the end of this. Many reactions move to their conclusion and then stop,. it is also possible to achieve chemical equilibrium in a reaction involving. Equilibrium Chemistry Real Life Examples.
From www.youtube.com
Dynamic Equilibrium Real Life Examples YouTube Equilibrium Chemistry Real Life Examples Reactions are the verbs of chemistry—the activity that chemists study. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. Many reactions move to their conclusion and then stop,. a reaction is at equilibrium when the amounts of reactants or products no longer change. it is. Equilibrium Chemistry Real Life Examples.
From teachkidschemistry.com
Equilibrium Teach Kids Chemistry Equilibrium Chemistry Real Life Examples to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. a reaction is at equilibrium when the amounts of reactants or products no longer change. Reactions are the verbs of chemistry—the activity that chemists study. An example of such heterogeneous equilibrium. when a reaction system is. Equilibrium Chemistry Real Life Examples.
From www.chemistrylearner.com
Chemical Equilibrium Definition, Principles, and Examples Equilibrium Chemistry Real Life Examples Graphs derived by plotting a few equilibrium concentrations for a system at a given temperature and pressure. The concept of chemical equilibrium was developed in 1803, after berthollet found that some chemical. Chemical equilibrium is a dynamic process, meaning the rate of. a reaction is at equilibrium when the amounts of reactants or products no longer change. it. Equilibrium Chemistry Real Life Examples.
From www.len.com.ng
Chemical Equilibrium Dynamic Equilibrium in Chemistry Equilibrium Chemistry Real Life Examples Many reactions move to their conclusion and then stop,. a reaction is at equilibrium when the amounts of reactants or products no longer change. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. An example of such heterogeneous equilibrium. Graphs derived by plotting a few equilibrium concentrations. Equilibrium Chemistry Real Life Examples.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Chemistry Real Life Examples Chemical equilibrium is a dynamic process, meaning the rate of. Many reactions move to their conclusion and then stop,. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. a reaction is at equilibrium when the amounts of reactants or products no longer change. The concept of chemical. Equilibrium Chemistry Real Life Examples.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free Equilibrium Chemistry Real Life Examples it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. when a reaction system is at equilibrium, q = k. An example of such heterogeneous equilibrium. Reactions are the verbs of chemistry—the activity that chemists study. Chemical equilibrium is a dynamic process, meaning the rate of. to. Equilibrium Chemistry Real Life Examples.
From printablelibtesty.z13.web.core.windows.net
How To Understand Chemical Equilibrium Equilibrium Chemistry Real Life Examples By the end of this. An example of such heterogeneous equilibrium. when a reaction system is at equilibrium, q = k. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. Reactions are the verbs of chemistry—the activity that chemists study. it is also possible to. Equilibrium Chemistry Real Life Examples.
From www.youtube.com
Importance of Equilibrium Constant Part 1 Lecture 06 Chapter 09 Equilibrium Chemistry Real Life Examples By the end of this. Chemical equilibrium is a dynamic process, meaning the rate of. Graphs derived by plotting a few equilibrium concentrations for a system at a given temperature and pressure. An example of such heterogeneous equilibrium. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. Reactions. Equilibrium Chemistry Real Life Examples.
From facts.net
10 Captivating Facts About Chemical Equilibrium Equilibrium Chemistry Real Life Examples The concept of chemical equilibrium was developed in 1803, after berthollet found that some chemical. An example of such heterogeneous equilibrium. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. a reaction is at equilibrium when the amounts of reactants or products no longer change. Many reactions. Equilibrium Chemistry Real Life Examples.
From www.youtube.com
Chemical equilibrium with real examples YouTube Equilibrium Chemistry Real Life Examples to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. By the end of this. when a reaction system is at equilibrium, q = k. a reaction is at equilibrium when the amounts of reactants or products no longer change. Graphs derived by plotting a few. Equilibrium Chemistry Real Life Examples.
From gamma.app
Exploring Equilibrium RealLife Examples. Equilibrium Chemistry Real Life Examples Chemical equilibrium is a dynamic process, meaning the rate of. a reaction is at equilibrium when the amounts of reactants or products no longer change. An example of such heterogeneous equilibrium. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. Graphs derived by plotting a few. Equilibrium Chemistry Real Life Examples.
From www.shutterstock.com
Chemical Equilibrium Infographic Diagram Showing Lab Stock Illustration Equilibrium Chemistry Real Life Examples By the end of this. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. Graphs derived by plotting a few equilibrium concentrations for a system at a given temperature and pressure. when a reaction system is at equilibrium, q = k. a reaction is at equilibrium. Equilibrium Chemistry Real Life Examples.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium Equilibrium Chemistry Real Life Examples An example of such heterogeneous equilibrium. The concept of chemical equilibrium was developed in 1803, after berthollet found that some chemical. Chemical equilibrium is a dynamic process, meaning the rate of. a reaction is at equilibrium when the amounts of reactants or products no longer change. Reactions are the verbs of chemistry—the activity that chemists study. it is. Equilibrium Chemistry Real Life Examples.
From www.youtube.com
What is Chemical Equilibrium Explain with Example Class 10 Chemistry Equilibrium Chemistry Real Life Examples Chemical equilibrium is a dynamic process, meaning the rate of. An example of such heterogeneous equilibrium. when a reaction system is at equilibrium, q = k. Many reactions move to their conclusion and then stop,. a reaction is at equilibrium when the amounts of reactants or products no longer change. Reactions are the verbs of chemistry—the activity that. Equilibrium Chemistry Real Life Examples.
From lawofthermodynamicsinfo.com
What Is Thermodynamic Equilibrium? (With Best Examples) Equilibrium Chemistry Real Life Examples when a reaction system is at equilibrium, q = k. a reaction is at equilibrium when the amounts of reactants or products no longer change. By the end of this. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single. An example of such heterogeneous equilibrium.. Equilibrium Chemistry Real Life Examples.
From brainly.in
What is meant by Dynamic Equilibrium? Brainly.in Equilibrium Chemistry Real Life Examples Many reactions move to their conclusion and then stop,. Reactions are the verbs of chemistry—the activity that chemists study. when a reaction system is at equilibrium, q = k. it is also possible to achieve chemical equilibrium in a reaction involving substances in more than one phase of matter. to describe how to calculate equilibrium concentrations from. Equilibrium Chemistry Real Life Examples.